As lubricant industry is seeking for more environmental, cost-effective and reliable test methods, scientists and concerned industry peers collaborate on developing solutions that will serve the needs of the industry.
In this article Professor Emeritus and former Chair of the Department of Food Science and Agricultural Chemistry at McGill University Dr. Frederick van de Voort approaches FTIR spectroscopy and explains the essence of the methodology.
A new approach to the determination of Base Number (BN) by FTIR spectroscopy is starting to gain traction in the lubricant sector. A manual FTIR BN method designed to produce ASTM titrimetric-similar BN results has been developed and validated, the details having been recently published in Lubrication Science (1). Service and on-site laboratories can achieve significant savings in time, labor and reduce their environmental/maintenance footprint relative to titration and avoid IR systems employing questionable oil-dependent correlational chemometric calibrations. Using a simple, universal Beer’s Law calibration, independent of oil type/condition it specifically targets the acid-base reaction much like titration. Based on the analysis of new/in-service oils analyzed by both FTIR and ASTM D4739 the method was demonstrated to match up well, accurate to within ~± 1.0 BN over a range of 10 BN and a within-oil reproducibility of ~±0.50 BN.
The McGill IR group has been at the forefront of developing automated quantitative methods for the analysis of in-use oils by FTIR spectroscopy with a focus on automated methods (2). In collaboration with Lubetech (Australia) which operates a commercial in-service lubricant analysis laboratory, a generic FTIR BN method was developed and validated. Their interest evolved from an earlier Canadian/Australian collaboration to work towards developing manual AN and BN FTIR methods (3), as implemented on an Agilent 5500t FTIR. The objective was to develop generic FTIR AN and BN methods able to produce titrimetric-comparable AN or BN analytical results without the need to invest in more sophisticated, fully automated systems, yet still achieve superior analytical rates to facilitate on-site analysis where reliable BN information is critical.
FTIR Spectroscopy and Calibration
An Agilent open-architecture TumblIR® accessory (nominal pathlength ~100 m) µ available for the Agilent 5500 FTIR Series has been demonstrated to be particularly convenient for BN analysis, specifically in terms of sample handling, requiring minimal sample preparation (~1.5 ml) and only a drop or two for spectral analysis.

Figure 1: TumblIR accessory for sample handling
Although optimal, a simple demountable transmission flow cell loaded by aspiration can also be used so that any standard FTIR can be utilized, albeit requiring more sample (~10 ml) to load and rinse out previous samples. Calibration and analysis are particularly simple, relying only on a certified Conostan® BN standard diluted with mineral oil to produce a series of BN standards. Sample analysis as presented in Figure 2 is straight forward, with the oil to be analysed split into two 0.5 ml portions conveniently placed in 2 ml microfuge tubes and having a reagent-free diluent blank (Do) and a reagent-containing diluent containing trifluoroacetic acid (DTFA) added to each, respectively.
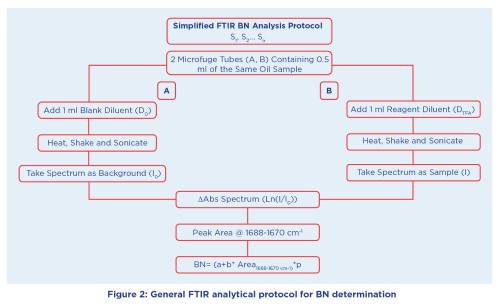
The sample and diluent are not miscible but are thoroughly mixed and placed in a sonic bath to ensure the reaction/extraction is complete. Spectral analysis consists of taking the spectra of the Do and DTFA sequentially to produce a differential absorbance
spectrum (ΔAbs) which ratios out the common oil components, but leaves the spectral changes specifically related to the acid-base reaction to be measured. The calibration and analysis are based on the formation of the TFA salt (COO-) upon reacting with base in the oil, the spectral signal change (area or peak height) being proportional to the BN of the sample as shown in the calibration plot (Figure 3).
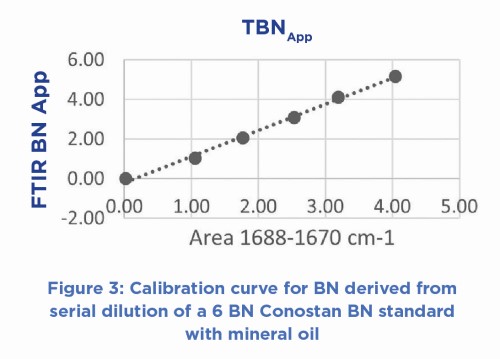
The calibration relationship derived has an SD of ± 0.151 BN which compares well with ±0.161 BN for the dilution of a new oil with mineral oil and analyzed by ASTM 4739. In a head-to-head comparison, 36 lubricants, comprising of both new and used oils were analysed by both methods, the results illustrated in Figure 3.
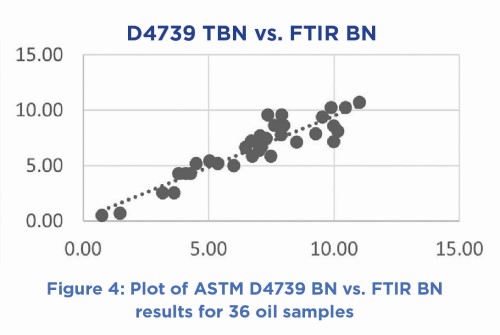
The two methods are linearly related, having a slope of 0.92 and an intercept near zero.
ASTM D4739 = 0.9265*FTIR BN + 0.2678 R2 = 0.8493 SD = ±1.005
This relationship indicates that one can measure BN to within ~1.0 BN of what one would expect to determine by ASTM 4739 potentiometric titration. It is clear from a more detailed comparative statistical assessment that both methods track the BN changes well, expected given that they have a similar stoichiometric basis; hence the term ASTM-titrimetric-comparable FTIR results.
Discussion
The FTIR BN results obtained are similar in magnitude to those of D4739 even though TFA is a substantially weaker acid than HCl, attributed to TFA being present at ~6-fold greater concentration than the 0.10 N HCl used in D4739. A key benefit of the FTIR method is that it is a split-sample method, the calibration and analytical results being completely independent of oil-type as it targets spectral changes specific to the acid-base reaction. Thus, the simple Beer’s Law calibration devised is universal and readily validated. This is not the case with FTIRs and filter-based IRs that predict BN from neat oils using chemometrically derived Library calibrations (4). These correlational calibrations are strongly oil-type dependent, do not target a unique BN spectral signal and are difficult to validate, relying solely on the manufacturer’s claims of performance. As such, the results obtained can be problematic to rely on, especially if the oil is not represented, identified properly, or topped up with the wrong oil-type. Until now, no such simple, alternate means of obtaining reliable BN data has been available for making critical machine reliability decisions. As such, this new methodology provides smaller in-service laboratories and on-site users with a means of obtaining accurate BN data with FTIR equipment most have already in place.
Conclusion
This brief article only serves as a general overview of the essence of the methodology. Details of a companion FTIR AN method have recently been published in the Proceedings of an ASTM Symposium in Support of the Lubricant Condition Monitoring Industry (5). A comprehensive review of the evolution quantitative FTIR analysis is also available in Tribology OnLine (6). Both the AN and BN methods can be implemented on most FTIRs commonly found in lubricant service laboratories and share simple sample preparation, minimal reagents/solvents and spectral analysis takes ~1 minute after sample pairs have been prepared and reacted. This contrasts to the 30-45 minutes per sample for ASTM potentiometric titration which has a significant environmental and maintenance footprint. Lubetech has implemented this FTIR BN analysis as an option for their clients as a more cost-effective means of providing reliable titrimetric-similar results serving the determinative purpose of continuing to use an oil or to condemning it to safeguard machinery reliability.

Frederick R. van de Voort
Professor Emeritus and former Chair of the Department of Food Science and Agricultural Chemistry at McGill University, where he co-directed the McGill IR Group, focusing on developing rapid quantitative FTIR methods for the analysis of edible oils and lubricants.




